Cognoa’s AI platform for autism diagnosis gets first FDA stamp
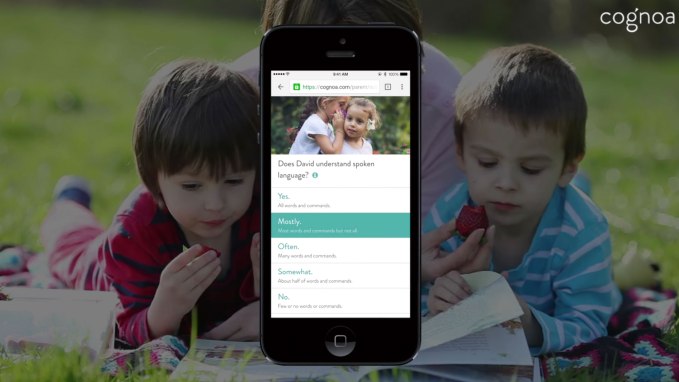
 Cognoa has gained regulatory recognition for its machine learning software as a class II diagnostic medical device for autism — meaning the digital health startup is now positioned to submit an application for full FDA clearance. Read More
Cognoa has gained regulatory recognition for its machine learning software as a class II diagnostic medical device for autism — meaning the digital health startup is now positioned to submit an application for full FDA clearance. Read More
No comments